F22 Delusional Disorder Somatic Type Continuous With Psychosis
Delusional parasitosis is characterised by the fixed belief that one is infested with parasites or small living creatures although there is no medical evidence for this (Reference ArnoldArnold, 2000; Reference FreudenmannFreudenmann, 2002). Patients usually complain about itching that they ascribe to the presence of animals in or under the skin. The belief is usually held with delusional intensity but the severity of the delusional intensity can vary. The annual prevalence of delusional parasitosis is estimated at 80 cases per million inhabitants, with a yearly incidence of 20 per million (Reference TrabertTrabert, 1997).
History
It is usually said that delusional parasitosis was first described by Thibierge in 1894 and Perrin in 1896. However, according to Trabert's comprehensive historical literature search (Trabert, Reference Trabert1993, Reference Trabert1997), the clinical picture was first mentioned by Robert Willan in 1799 and Johann Heinrich Jördens in 1801. According to our research neither author suspected a psychiatric aetology. Moreover, Musalek (Reference Musalek1991) discovered a patient with delusions of intestinal parasitosis (Enterozoenwahn) in an article from 1843 (Reference CharcellayCharcellay, 1843).
Pathogenesis
Delusional parasitosis is a non-specific syndrome rather than a single disorder. It can occur as a delusional disorder, meeting ICD–10 criteria for persistent delusional disorder (World Health Organization, 1993) and DSM–IV–TR criteria for delusional disorder, somatic type (American Psychiatric Association, 2000). This is what clinicians usually mean when they refer to 'delusional parasitosis', although it accounts only for about 40% of all patients with such symptoms (Reference TrabertTrabert, 1995). We will call this type of delusional parasitosis 'primary delusional parasitosis' following Ganner & Lorenzi's (Reference Ganner and Lorenzi1975) concept of 'reiner Dermatozoenwahn' (German 'rein'=primary or pure). The diagnosis of primary delusional parasitosis can be made only after real infection or other underlying medical or psychiatric conditions have been excluded, because delusional parasitosis can be associated with several physical illnesses, psychiatric disorders or intoxications (Reference Magnan and SauryMagnan & Saury, 1889; Reference EkbomEkbom, 1938; Reference HuberHuber, 1957; Reference BerriosBerrios, 1985; Reference Freyne and WrigleyFreyne & Wrigley, 1994; Reference FreudenmannFreudenmann, 2002). Delusional parasitosis can also occur as a folie à deux or folie à trois (shared psychotic disorder; Reference TrabertTrabert, 1995) as well as by proxy (Reference Nel, Schoeman and LobettiNel et al, 2001). Delusional parasitosis syndromes can thus be classified according to their pathogenesis (Appendix 1).
Clinical management
The clinical management of patients with delusional parasitosis is a challenge, as patients are often reluctant to engage in a meaningful therapeutic relationship because of their somatic concept of the illness. Thus they seek help from general practitioners, dermatologists or pest control companies but refuse psychiatric referral or therapy. Usually, it is difficult to obtain informed consent to treat patients with delusional parasitosis with antipsychotics. Therefore experienced clinicians tell their patients that the antipsychotics are effective 'against the itch' or the 'problems with the pests' in order not to have to lie. A few practical guidelines have been proposed (Reference MusalekMusalek, 1991; Reference Driscoll, Rothe and Grant-KelsDriscoll et al, 1993; Reference WinstenWinsten, 1997; Reference FreudenmannFreudenmann, 2002).
Another approach to achieve a better therapeutic relationship was developed in the late 1980s. Specialised out-patient clinics were located in dermatology clinics to acknowledge the patients' non-psychiatric concept of their illness (Reference Musalek and KutzerMusalek & Kutzer, 1989; Reference Musalek, Bach and GerstbergerMusalek et al, 1989; Reference MusalekMusalek, 1991; Reference TrabertTrabert, 1993). However, even these 'low threshold' settings have often failed to allow the establishment of a sufficient therapeutic alliance. Trabert's study in Homburg, Germany stated that 20 of 35 patients (57%) were seen for less than 3 months (Reference TrabertTrabert, 1993). Despite all these efforts, many patients lose faith in professional medicine and resort to dangerous self-therapies such as excessive skin cleaning with chemicals or pesticides (Reference FreudenmannFreudenmann, 2002).
Antipsychotic treatment
For adequate treatment of delusional parasitosis it is necessary to differentiate between the different forms (Reference BerriosBerrios, 1985; Reference Freudenmann and Schönfeldt-LecuonaFreudenmann & Schönfeldt-Lecuona, 2005). Although antipsychotics provide the main treatment for primary delusional parasitosis, they are used only symptomatically for delusional parasitosis secondary to somatic diseases, which mainly requires adequate therapy of the underlying disorder. Even in recent years, many sources recommended the use of the typical antipsychotic pimozide in delusional parasitosis (Reference Driscoll, Rothe and Grant-KelsDriscoll et al, 1993; Reference van Vlotenvan Vloten, 2003), although pimozide is no longer a first-line antipsychotic because of concerns about drug safety (high risk of extrapyramidal symptoms, longer QTc interval and drug–drug interactions (Food and Drug Administration, 1996; National Institute for Clinical Excellence, 2002; Reference Benkert and HippiusBenkert & Hippius, 2005).
Several case reports have indicated the beneficial effects of atypical antipsychotics in primary delusional parasitosis, but evidence for these is still limited to risperidone (Reference Gallucci and BeardGallucci & Beard, 1995; Reference Freyne, Kenny and CooneyFreyne et al, 1999; Reference Moretti and VargaMoretti & Varga, 2000), quetiapine (Reference Kim, Kim and LeeKim et al, 2003), olanzapine (Reference Le and GonskiLe & Gonski, 2003) and amisulpride (Reference Lepping, Gil-Candon and FreudenmannLepping et al, 2005).
Although it is often stated that there is a lack of randomised controlled trials of the use of antipsychotics (including pimozide) in delusional parasitosis (Reference Driscoll, Rothe and Grant-KelsDriscoll et al, 1993; Reference TrabertTrabert, 1995; Reference Freudenmann and Schönfeldt-LecuonaFreudenmann & Schönfeldt-Lecuona, 2005), we know of no systematic review on this topic. Moreover, no antipsychotic is licensed for the treatment of delusional parasitosis. We therefore undertook the first systematic review of the effectiveness of typical and atypical antipsychotic treatment for primary delusional parasitosis (meeting ICD–10 F22.0 criteria for persistent delusional disorder or DSM–IV–TR criteria for delusional disorder of somatic type) in order to determine whether: typical antipsychotics are effective in treating primary delusional parasitosis and are more effective than placebo; atypical antipsychotics are effective in treating primary delusional parasitosis and more effective than placebo; atypical antipsychotics are more or less effective than typical antipsychotics.
METHODS
Search strategy
Our first priority was to discover randomised controlled trials that addressed the study questions. We tried to identify all available works on delusional parasitosis published in English, German, French, Spanish, Portuguese, Italian, Dutch or Hungarian before December 2005. A comprehensive search of EMBASE, Medline, PsycInfo, PsycLit and Psyndex was performed using the search terms 'delusion(s) of parasitosis', 'delusional parasitosis', 'delusion(s) of infestation', '*parasitosis*', 'monosymptomatic hypochondriacal psychosis', 'parasitophob*', 'entomophob*', 'acarophob*', 'Dermatozoenwahn', and 'Ekbom's syndrome' (discarding papers on the 'burning feet syndrome' which has also been labelled with this eponym). We checked the reference lists of identified articles. We searched the internet using Google, and textbooks of psychiatry, theses, unlisted journals and conference proceedings by hand. We wrote to pharmaceutical companies producing substances often used to treat delusional parasitosis and known authorities in the field of delusional parasitosis (e.g. Wolfgang Trabert, Emden, Germany, and Marc Bourgeois, Bordeaux, France) to identify unpublished data on the use of antipsychotics in delusional parasitosis. We searched for continuing trials via two websites – Clinical Trials (http://www.clinicaltrials.gov) and Current Controlled Trials (http://www.controlledtrials.com).
Assessment of literature
P.L. and R.W.F. independently assessed whether all retrieved works dealt with delusional parasitosis in general or primary delusional parasitosis, and whether the intervention consisted of typical or atypical antipsychotics. We also assessed whether any of these were randomised controlled trials.
In the absence of randomised controlled trials we planned to gather sound evidence from other studies meeting defined inclusion criteria (see Appendix 2). In particular we sought well-designed quasi-experimental and observational studies relevant to our research questions. We included all open studies with either prospective design or more than 30 patients. We then summarised in structured form the main findings of the 16 studies meeting these minimal criteria (Table DS1, data supplement to online version of this paper). We assigned individual outcomes between three main categories: no effect (0); partial remission (i.e. some response) (1) and full remission (2). To strengthen our conclusions we also tried to separate primary delusional parasitosis from secondary delusional parasitosis.
P.L. and R.W.F. also selected all case reports containing information on diagnosis of primary delusional parasitosis, gender, age, antipsychotic medication used and dose, and clinical outcome on the same 3-point scale after 4 weeks or more. In this way we applied Trabert's case-based meta-analysis which is designed for uncommon syndromes that cannot be studied in a traditional randomised controlled trial (Reference TrabertTrabert, 1995). We also separated patients treated with typical and atypical antipsychotics. Although this approach is subject to publication bias because it considers only published cases, we aimed to increase comparability between studies. To judge the success of this strategy we tested whether clinical outcomes differed significantly between studies.
RESULTS
Literature search
We identified a total of 368 works on delusional parasitosis in general, including poster presentations, in December 2005 (Fig. 1). We found most on Medline (n=191), and in review articles and the comprehensive theses of Musalek (Reference Musalek1991) and Trabert (Reference Trabert1993). At least half were not in English – most of these were in German, but some were in Italian and French. The full bibliography can be obtained from the authors on request.

Fig. 1 Method used for identification and selection of studies of delusional parasitosis.
Before the psychopharmacological era, which began with the discovery of chlorpromazine in 1952, only 31 works on delusional parasitosis were retrieved. The majority (n=223) were published between 1952 and the launch of risperidone in about 1990. The remainder (n=114) were published after 1990, but many of these did not examine the use of atypical antipsychotics.
Absence of randomised controlled trials
Our systematic search found no randomised controlled trials on the effects of typical or atypical antipsychotics in either primary or other delusional parasitosis. When accessed in December 2005, the Clinical Trials (http://www.clinicaltrials.gov) and Current Controlled Trials (http://www.controlled-trials.com) websites gave no indication of unpublished or current randomised controlled trials. Our evaluation of the literature therefore relied on results from other studies.
Effect of typical antipsychotics
Table DS1 (see data supplement to online version of this paper) summarises the 16 quasi-experimental or observational studies which primarily used typical antipsychotics and met our inclusion criteria (Reference FrithzFrithz, 1979; Reference Hamann and AvnstorpHamann & Avnstorp, 1982; Reference MunroMunro, 1982; Reference LyellLyell, 1983; Ungvari, Reference Ungvari1983, Reference Ungvari1984; Ungvari & Vladar, Reference Ungvari and Vladar1984, Reference Ungvari and Vladar1986; Reference Lindskov and BaadsgaardLindskov & Baadsgaard, 1985; Reference Bourgeois, Rager and PeyreBourgeois, et al, 1986; Reference Reilly and BatchelorReilly & Batchelor, 1986; Reference Musalek, Bach and GerstbergerMusalek, et al, 1989; Reference PaholpakPaholpak, 1990; Trabert, Reference Trabert1993, Reference Trabert1995; Reference Srinivasan, Suresh and JayaramSrinivasan et al, 1994; Reference Zomer, De Wit and Van BronswijkZomer et al, 1998; Reference Bhatia, Jagawat and ChoudharyBhatia et al, 2000). The studies showed aggregate partial and full remission rates between 60 and 100% after treatment with typical antipsychotics. Unfortunately, the majority were not limited to primary delusional parasitosis. Nevertheless they suggest a generally good outcome for primary and other forms of delusional parasitosis whenever continuous antipsychotic treatment can be established.
In primary delusional parasitosis, aggregate partial and full remission rates with pimozide ranged from 67 % (n=66; Reference LyellLyell, 1983) through 89% (n=9; Reference MunroMunro, 1982) to 100% (n=10; Ungvari & Vladar, Reference Ungvari and Vladar1984, Reference Ungvari and Vladar1986), n=18; Ungvari, Reference Ungvari1983, Reference Ungvari1984). In mixed samples with primary and other forms of delusional parasitosis, aggregate partial and full remission rates with pimozide were similar and varied from 61% (n=33; Reference Zomer, De Wit and Van BronswijkZomer et al, 1998), through 87% (n=52; Reference Bhatia, Jagawat and ChoudharyBhatia et al, 2000) to 91% (n=11; Reference Hamann and AvnstorpHamann & Avnstorp, 1982). A high rate of side-effects such as sedation, extrapyramidal symptoms and depression was noted in several studies using pimozide (38%; Ungvari, Reference Ungvari1983, Reference Ungvari1984 and 73%; Reference Hamann and AvnstorpHamann & Avnstorp, 1982).
The only two placebo-controlled trials in delusional parasitosis both used pimozide (Reference Hamann and AvnstorpHamann & Avnstorp, 1982; Ungvari & Vladar, Reference Ungvari and Vladar1984, Reference Ungvari and Vladar1986). However, they are limited by a lack of randomised allocation to the treatment groups and small samples (n=10 or 11 respectively). Only Ungvari & Vladar (Reference Ungvari and Vladar1984, Reference Ungvari and Vladar1986) treated patients with primary delusional parasitosis.
Studies not specific for particular antipsychotics demonstrated aggregate partial and full remission rates between 82% (n=35, not only primary delusional parasitosis, Reference TrabertTrabert, 1993) and 89% (n=19, only primary delusional parasitosis, but some patients were treated with electroconvulsive therapy; Reference Srinivasan, Suresh and JayaramSrinivasan et al, 1994).
The only study that investigated traditional depot antipsychotics found an aggregate response and remission rate of 93%, even in patients that could not be treated with oral medication (Reference FrithzFrithz, 1979).
The sample consisted only of patients with primary delusional parasitosis (n=15). Another study using haloperidol reported a 100% response rate (but no full remissions) (Reference PaholpakPaholpak, 1990); nine of ten patients in this study had primary delusional parasitosis.
Across the different typical antipsychotics used, an effect of antipsychotic medication in delusional parasitosis was noted after about 3–6 weeks (Reference Hamann and AvnstorpHamann & Avnstorp, 1982; Reference TrabertTrabert, 1995). Studies came to different conclusions as to whether or not it is necessary to continue antipsychotics after successful acute therapy.
Symptoms of delusional parasitosis that were associated with major depression could be treated successfully with antidepressants (Reference Musalek, Bach and GerstbergerMusalek et al, 1989; Reference TrabertTrabert, 1993; Reference Bhatia, Jagawat and ChoudharyBhatia et al, 2000).
One small study indicated that electroconvulsive therapy might be effective in patients with primary delusional parasitosis (Reference Srinivasan, Suresh and JayaramSrinivasan et al, 1994).
A survey of British dermatologists suggested that combining psychopharmacological and dermatological treatments (local and systemic) is superior to single therapeutic approaches. Of particular note is that therapy without psychotropic medication was ineffective (Reference Reilly and BatchelorReilly & Batchelor, 1986).
We abstracted data from case series and case reports on 92 patients with primary delusional parasitosis treated with typical antipsychoticss who met our selection criteria – (see Table DS2 (data supplement to online version of this paper) (Reference Riding and MunroRiding & Munro, 1975; Reference Gould and GraggGould & Gragg, 1976; Munro, Reference Munro1978a ,Reference Munro b , Reference Munro1982; Reference FrithzFrithz, 1979; Reference Avnstorp, Hamann and JepsenAvnstorp et al, 1980; Reference UngvariUngvari, 1984; Reference BerriosBerrios, 1985; Reference Andrews, Bellard and Walter-RyanAndrews et al, 1986; Reference Sheppard, O'Loughlin and MaloneSheppard et al, 1986; Reference Ungvari and VladarUngvari & Vladar, 1986; Reference May and TerpenningMay & Terpenning, 1991; Reference Srinivasan, Suresh and JayaramSrinivasan et al, 1994; Reference Chiu, Ungvari and WongChiu et al, 1996; Reference Räsänen, Erkonen and IsakssonRasanen et al, 1997; Reference Kim, Kim and LeeKim et al, 2003; Reference Takahashi, Ozawa and InuzukaTakahashi et al, 2003).
Table 1 shows that the majority were treated with pimozide, whereas for other typical antipsychotics only small numbers have been reported. The majority of these patients with primary delusional parasitosis were effectively treated with typical antipsychotics. Pimozide was helpful for 50 of 53 patients (94%), with 24 (45%) achieving full remission. Trifluoperazine and haloperidol appeared to be similarly effective, as did fluphenazine and flupenthixol depot.
Table 1 Summary of 92 case reports of the treatment of primary delusional parasitosis with typical antipsychotics 1
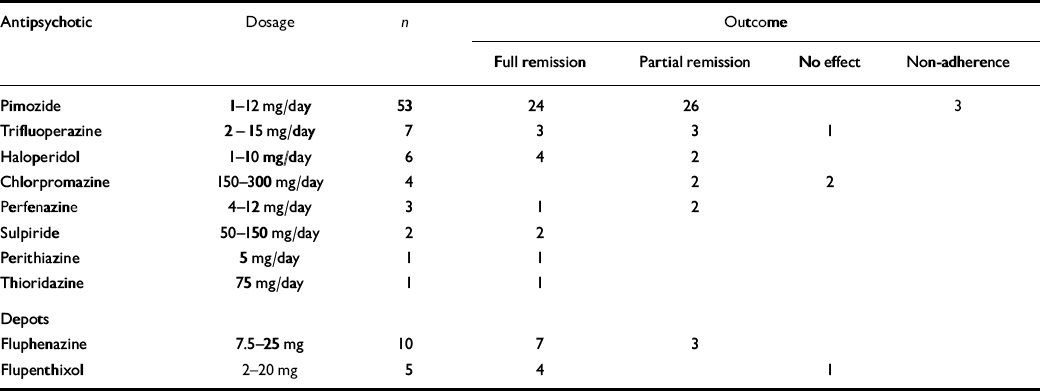
| Antipsychotic | Dosage | n | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Full remission | Partial remission | No effect | Non-adherence | ||||||
| Pimozide | 1-12 mg/day | 53 | 24 | 26 | 3 | ||||
| Trifluoperazine | 2 - 15 mg/day | 7 | 3 | 3 | 1 | ||||
| Haloperidol | 1-10 mg/day | 6 | 4 | 2 | |||||
| Chlorpromazine | 150-300 mg/day | 4 | 2 | 2 | |||||
| Perfenazine | 4-12 mg/day | 3 | 1 | 2 | |||||
| Sulpiride | 50-150 mg/day | 2 | 2 | ||||||
| Perithiazine | 5 mg/day | 1 | 1 | ||||||
| Thioridazine | 75 mg/day | 1 | 1 | ||||||
| Depots | |||||||||
| Fluphenazine | 7.5-25 mg | 10 | 7 | 3 | |||||
| Flupenthixol | 2-20 mg | 5 | 4 | 1 | |||||
Effect of atypical antipsychotics
We were able to identify only 12 case reports on atypical antipsychotics that met our selection criteria (Table 2). Although atypical antipsychotics were effective for the majority of patients with primary delusional parasitosis only three patients achieved full remission. All six patients for whom risperidone was prescribed, achieved full (four) or partial (two) remission; the dosages used ranged from 1 to 8 mg per day.
Table 2 Summary of 12 case reports of the treatment of primary delusional parasitosis with atypical antipsychotics
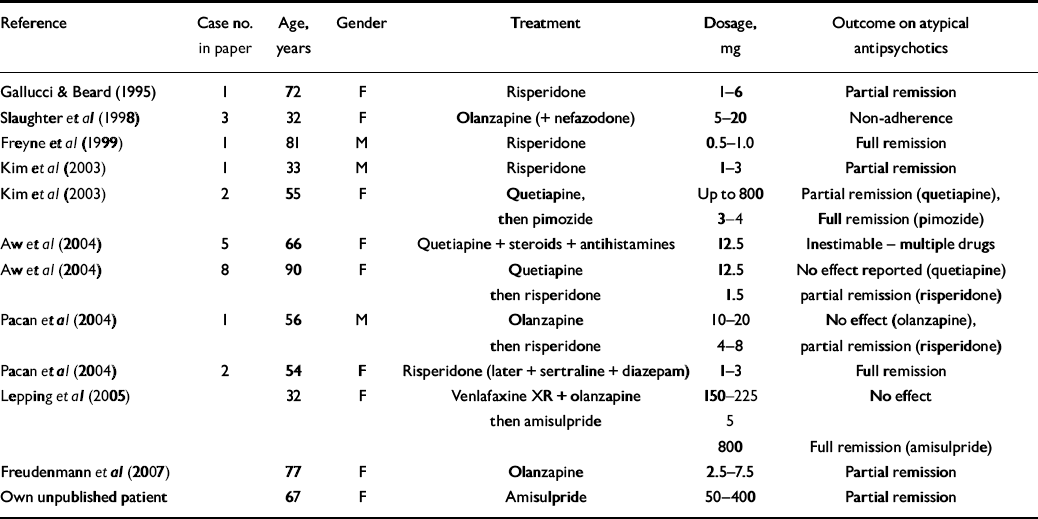
| Reference | Case no. in paper | Age, years | Gender | Treatment | Dosage, mg | Outcome on atypical antipsychotics |
|---|---|---|---|---|---|---|
| Gallucci & Beard (Reference Gallucci and Beard1995) | 1 | 72 | F | Risperidone | 1-6 | Partial remission |
| Slaughter et al (Reference Slaughter, Zanol and Rezvani1998) | 3 | 32 | F | Olanzapine (+ nefazodone) | 5-20 | Non-adherence |
| Freyne et al (Reference Freyne, Kenny and Cooney1999) | 1 | 81 | M | Risperidone | 0.5-1.0 | Full remission |
| Kim et al (Reference Kim, Kim and Lee2003) | 1 | 33 | M | Risperidone | 1-3 | Partial remission |
| Kim et al (Reference Kim, Kim and Lee2003) | 2 | 55 | F | Quetiapine, then pimozide | Up to 800 3-4 | Partial remission (quetiapine), Full remission (pimozide) |
| Aw et al (Reference Aw, Thong and Chan2004) | 5 | 66 | F | Quetiapine + steroids + antihistamines | 12.5 | Inestimable - multiple drugs |
| Aw et al (Reference Aw, Thong and Chan2004) | 8 | 90 | F | Quetiapine then risperidone | 12.5 1.5 | No effect reported (quetiapine) partial remission (risperidone) |
| Pacan et al (Reference Pacan, Reich and Szepietowski2004) | 1 | 56 | M | Olanzapine then risperidone | 10-20 4-8 | No effect (olanzapine), partial remission (risperidone) |
| Pacan et al (Reference Pacan, Reich and Szepietowski2004) | 2 | 54 | F | Risperidone (later + sertraline + diazepam) | 1-3 | Full remission |
| Lepping et al (Reference Lepping, Gil-Candon and Freudenmann2005) | 32 | F | Venlafaxine XR + olanzapine then amisulpride | 150-225 5 | No effect | |
| 800 | Full remission (amisulpride) | |||||
| Freudenmann et al (Reference Freudenmann, Schönfeldt-Lecuona and Lepping2007) | 77 | F | Olanzapine | 2.5-7.5 | Partial remission | |
| Own unpublished patient | 67 | F | Amisulpride | 50-400 | Partial remission |
Effect of typical v. atypical antipsychotics
The five main studies of patients with primary delusional parasitosis treated with typical antipsychotics report very heterogeneous outcomes (Table 3), suggesting that the studies themselves are very heterogeneous, for example in their inclusion and exclusion criteria (selection bias) or in their definitions of partial and full remission (measurement bias), or that there is publication bias. Furthermore, the eight studies of patients with secondary delusional parasitosis treated with typical antipsychotics reported heterogeneous outcomes. This reduces the value of comparing studies reporting the use of typical primary antipsychotics in secondary delusional parasitosis. However, there was a large difference between the failure rate of 5% in primary delusional parasitosis and that of at least 26% in secondary delusional parasitosis (χ2=18.2, d.f.=2, P<0.001). It follows that studies of secondary delusional parasitosis have nothing to contribute to the issue of the most effective treatment for primary delusional parasitosis.
Table 3 Outcome of treatment of delusional parasitosis with antipsychotics
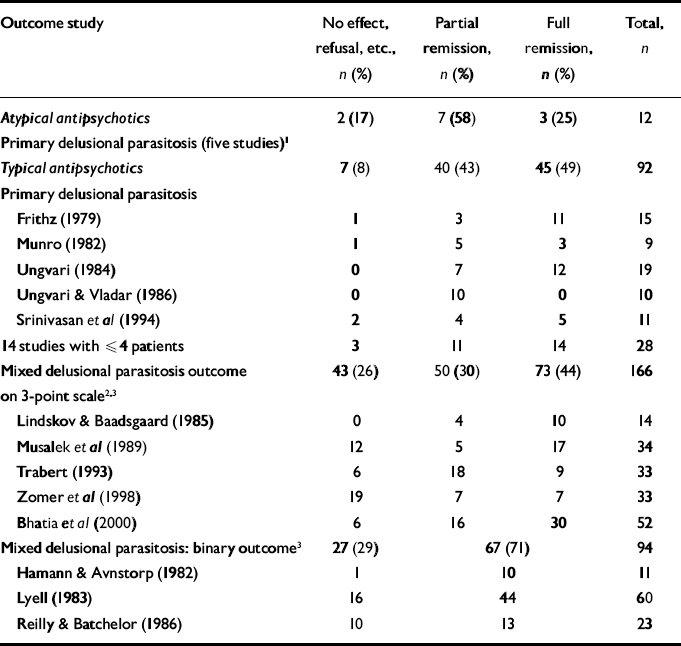
| Outcome study | No effect, refusal, etc., n (%) | Partial remission, n (%) | Full remission, n (%) | Total, n | |
|---|---|---|---|---|---|
| Atypical antipsychotics | 2 (17) | 7 (58) | 3 (25) | 12 | |
| Primary delusional parasitosis (five studies) 1 | |||||
| Typical antipsychotics | 7 (8) | 40 (43) | 45 (49) | 92 | |
| Primary delusional parasitosis | |||||
| Frithz (Reference Frithz1979) | 1 | 3 | 11 | 15 | |
| Munro (Reference Munro1982) | 1 | 5 | 3 | 9 | |
| Ungvari (Reference Ungvari1984) | 0 | 7 | 12 | 19 | |
| Ungvari & Vladar (Reference Ungvari and Vladar1986) | 0 | 10 | 0 | 10 | |
| Srinivasan et al (Reference Srinivasan, Suresh and Jayaram1994) | 2 | 4 | 5 | 11 | |
| 14 studies with ⩽4 patients | 3 | 11 | 14 | 28 | |
| Mixed delusional parasitosis outcome on 3-point scale 2 , 3 | 43 (26) | 50 (30) | 73 (44) | 166 | |
| Lindskov & Baadsgaard (Reference Lindskov and Baadsgaard1985) | 0 | 4 | 10 | 14 | |
| Musalek et al (Reference Musalek, Bach and Gerstberger1989) | 12 | 5 | 17 | 34 | |
| Trabert (Reference Trabert1993) | 6 | 18 | 9 | 33 | |
| Zomer et al (Reference Zomer, De Wit and Van Bronswijk1998) | 19 | 7 | 7 | 33 | |
| Bhatia et al (Reference Bhatia, Jagawat and Choudhary2000) | 6 | 16 | 30 | 52 | |
| Mixed delusional parasitosis: binary outcome 3 | 27 (29) | 67 (71) | 94 | ||
| Hamann & Avnstorp (Reference Hamann and Avnstorp1982) | 1 | 10 | 11 | ||
| Lyell (Reference Lyell1983) | 16 | 44 | 60 | ||
| Reilly & Batchelor (Reference Reilly and Batchelor1986) | 10 | 13 | 23 | ||
The heterogeneity in the reported outcome of patients with primary delusional parasitosis treated with typical antipsychotics reduces the value of comparison with reports of atypical antipsychotics. Although the difference is not statistically significant (χ2=2.6, d.f.=2), we cannot conclude that typical and atypical antipsychotics are equally effective because of the innate biases already identified.
DISCUSSION
This is the first systematic review of the effectiveness of typical and atypical antipsychotics in the treatment of delusional parasitosis. Our review was based on 368 published works and covered almost twice as many papers as the 193 covered by the most comprehensive review published to date (Reference TrabertTrabert, 1993). In contrast to previous review articles (e.g. Reference Aw, Thong and ChanAw et al, 2004; Reference Bourgeois and Nguyen-LanBourgeois & Nguyen-Lan, 1986; Reference Driscoll, Rothe and Grant-KelsDriscoll et al, 1993; Reference FreudenmannFreudenmann, 2002; Reference LynchLynch, 1993; Reference Slaughter, Zanol and RezvaniSlaughter et al, 1998; Reference WykoffWykoff, 1987; Reference Zanol, Slaughter and HallZanol et al, 1998), our review focuses on primary delusional parasitosis. We separated this important form of delusional parasitosis (delusional disorder of somatic type) from symptomatic forms of delusional parasitosis ('secondary delusional parasitosis') which cover different nosological entities and require other forms of therapy that focus on the underlying illness. As we were unable to show homogeneity in the outcomes of primary and secondary delusional parasitosis, we did not review other studies examining the effect of antipsychotic medication in other types of ICD–10 F22.0 disorders, which also differ clinically from delusional parasitosis.
Our systematic review identified no randomised controlled trials of the efficacy of typical and atypical antipsychotics in primary delusional parasitosis, probably because the disorder is rare and it is difficult to recruit patients, obtain informed consent, and achieve sufficient adherence to medication throughout a clinical trial because of their poor insight (Reference Gould and GraggGould & Gragg, 1976; Reference MunroMunro, 1982; Reference Ungvari and VladarUngvari & Vladar, 1986; Reference Freudenmann and Schönfeldt-LecuonaFreudenmann & Schönfeldt-Lecuona, 2005). It is difficult to establish a good therapeutic alliance with these patients even in highly specialised settings (Reference TrabertTrabert, 1993). Because of a lack of randomised controlled trials all our suggestions for therapy need to be interpreted cautiously. Nevertheless we are keen to provide clinicians with the current best evidence.
Use of typical antipsychotics
Our findings show that primary delusional parasitosis can be effectively treated with typical antipsychotics. Outcome is generally good, although this conclusion is limited by a possible publication bias. We confirm Trabert's finding that the introduction of typical antipsychotics has substantially improved remission rates (Reference TrabertTrabert, 1995). Although the better studies have so far used pimozide (Reference Hamann and AvnstorpHamann & Avnstorp, 1982; Ungvari & Vladar, Reference Ungvari and Vladar1984, Reference Ungvari and Vladar1986), the evidence for its efficacy is weak by today's standards. The level of evidence for its use in primary delusional parasitosis is IIa according to the criteria of the Agency for Health Care Policy and Research (1992), whereas other typical antipsychotics such as haloperidol have only level III evidence. Pimozide should not be used in patients with a high cardiac risk, together with other substances that prolong the QTc interval (Food and Drug Administration, 1996), or in elderly patients with delusional parasitosis.
Another important treatment in primary delusional parasitosis is the intramuscular application of traditional depot antipsychotics (Reference FrithzFrithz, 1979), because the main problem in clinical management is to convince patients to take oral medication regularly. Injection may be more consistent with patients' (false) somatic concept of their illness and require less cooperation than oral medication. If the patient agrees to a first depot injection, the delusion may well remit at least partially and further antipsychotic treatment will be accepted. As the only study of this approach (Reference FrithzFrithz, 1979) has only 15 patients, larger samples are needed.
Use of typical antipsychotics
Our systematic review revealed only 12 usable case reports of the use of atypical antipsychotics in primary delusional parasitosis. These provide limited evidence that primary delusional parasitosis can be treated effectively with these drugs. To our knowledge, this is the first complete collection of patients with primary delusional parasitosis treated with atypical antipsychotics, whereas many patients with secondary forms of delusional parasitosis have been reported in recent years (e.g. Reference De Leon, Furmaga and CanterburyDe Leon et al, 1997; Reference Safer, Wenegrat and RothSafer et al, 1997; Reference Kumbier and KornhuberKumbier & Kornhuber, 2002; Reference FreudenmannFreudenmann, 2003; Reference Le and GonskiLe & Gonski, 2003; Reference ScheinfeldScheinfeld, 2003; Reference Wenning, Davy and CatalanoWenning et al, 2003). Thus the evidence for the use of atypical antipsychotics in delusional parasitosis is even weaker than for typical antipsychotics.
Most case reports are available for risperidone, whereas we are not aware of reports on the use of clozapine, ziprasidone or aripiprazole in primary delusional parasitosis. Amisulpride might be a good alternative given that its selective D2/D3-antidopaminergic action resembles that of typical antipsychotics without the same high probability of side-effects (Reference Freudenmann and LeppingFreudenmann & Lepping, 2006; Reference Lepping, Gil-Candon and FreudenmannLepping et al, 2006). Its lack of anticholinergic and adrenolytic effects is particularly useful in elderly patients or patients with a higher cardiovascular risk profile. Risperidone microspheres for intramuscular injection provide a potentially interesting new treatment for delusional parasitosis, as this is the only atypical antipsychotic in depot form, but this recommendation is entirely theoretical since there are no reports of the use of risperidone microspheres in delusional parasitosis at present.
Other treatment options
An alternative to these pharmacological strategies is electroconvulsive therapy. The use of electroconvulsive therapy in a patient with delusional parasitosis was first described by Harbauer in 1949 and has since occasionally been reported (Reference BaumerBaumer, 1951; Reference Bers and ConradBers & Conrad, 1954; Reference HopkinsonHopkinson, 1970). Srinivasan et al (Reference Srinivasan, Suresh and Jayaram1994) reported effectiveness in primary delusional parasitosis in a small sample. Electroconvulsive therapy might be a useful option in cooperative refractory patients when antipsychotics are contraindicated or problematic (e.g. in the elderly).
Synthesis
The very heterogeneous outcomes reported by the five main studies of treating primary delusional parasitosis with typical antipsychotics (see Table 3) suggest that the studies themselves suffer from some or all of selection bias, measurement bias and publication bias. Together with these flaws the paucity of evidence on treating primary delusional parasitosis with atypical antipsychotics undermines any comparison of typical and atypical antipsychotics. Despite weaker evidence for the effectiveness of atypical antipsychotics in treating delusional parasitosis in comparison with pimozide, the use of atypical antipsychotics might improve side-effects, and thus adherence and patient outcome.
It is important to strengthen this weak evidence in the future. We limited our selection of case series and case reports of delusional parasitosis to those including a minimum data-set for each recruited patient (age, gender, the nature and timing of diagnosis, the name and dose of medication, and the nature and timing of remission on a 3-point scale; Appendix 2).
Implications
Our systematic review generated weak evidence that antipsychotics are effective in treating primary delusional parasitosis. However, in view of the limited evidence, this recommendation is tentative and needs caution in implementation. Since the introduction of atypical antipsychotics, pimozide is no longer the treatment of choice for reasons of drug safety, even though it has the best evidence of effectiveness in treating primary delusional parasitosis. It is important to improve this evidence through rigorous, ideally randomised, studies which compare typical antipsychotics and atypical antipsychotics directly.
APPENDICES
Appendix 1: Aetiological classification of delusional parasitosis
(I) Primary delusional parasitosis: delusional disorder.
Primary delusion according to Berrios (Reference Berrios1985), first described by Huber (Reference Huber1957); diagnosis: persistent delusional disorder (ICD-10 F22.0); delusional disorder, somatic type (DSM-IV-TR 297.1) Special form: as a shared psychotic disorder (ICD-10 F24, DSM-IV-TR 297.3)
(II) Secondary forms of delusional parasitosis: secondary to another condition.
-
(a) Concomitant psychotic symptom in another psychiatric disorder:
-
(1) Schizophrenia or other psychotic disorders; diagnosis according to underlying psychotic disorder (ICD-10 F2x, DSM-IV-TR 295, etc).
-
(2) Major depressive disorder with psychotic symptoms or mania; diagnosis according to underlying affective disorder (ICD-10 F3x, DSM-IV-TR 296, etc).
-
(3) Dementia; diagnosis ICD-10 F00-03, DSM-IV-TR 290, 294.
-
-
(b) Delusional parasitosis based on other brain pathologies ('macroscopic') or general medical condition:
-
(1) Brain disorders not mentioned in ICD-10 F0 (e.g. brain neoplasm/infection, stroke); 'organic damage with secondary delusions' according to Berrios (Reference Berrios1985), first described by Ekbom (Reference Ekbom1938).
-
(2) Somatic illness with pruritus or paraesthesia (e.g. diabetes mellitus with neuropathic pain, uraemia, jaundice, cancer); 'paraesthesia or other somatic pathological sensations with secondary delusions' according to Berrios (Reference Berrios1985); diagnosis: organic hallucinosis or organic delusional disorder (ICD-10 F06.0 or F06.2), psychotic disorder due to… [indicate the general medical condition] with delusions (DSM-IV-TR 293.81) or with hallucinations (293.82) or persistent delusional disorder (ICD-10 F22.0); delusional disorder, somatic type (DSM-IV-TR 297.1), when delusional parasitosis is not the direct physiological consequence of the somatic illness.
-
-
(c) Delusional parasitosis as a substance-induced 'toxic' psychosis:
Substance-induced 'paraesthesia or other somatic pathological sensations with secondary delusions' according to Berrios (Reference Berrios1985), first described by Magnan & Saury in 1889 for cocaine addicts ('signe de Magnan');
-
(1) Owing to psychotropic substance, e.g. cocaine, amphetamines; diagnosis: acute intoxication, psychotic disorder, predominantly delusional (ICD-10 F1x.51) or predominantly hallucinatory (ICD-10 F1x.52); substance-induced psychotic disorder, with hallucinations (DSM-IV-TR 292.11), with delusions (292.12).
-
(2) Owing to non-psychotropic substances, e.g. antibiotics, steroids, non-steroidal anti-inflammatory drugs; diagnosis: organic hallucinosis or organic delusional disorder (ICD-10 F06.0 or F06.2), DSM-IV-TR codes see 1.
-
Appendix 2: Proposed minimum information required for case series and case reports of primary delusional parasitosis
Case report criteria
-
(1) Diagnosis including confirmation date
-
(2) Gender of patient
-
(3) Age of patient
-
(4) Medication used with dosage
-
(5) Outcome on 3-point scale: (0) no remission, (1) partial remission, (2) full remission
-
(6) Length of follow-up (at least 4 weeks after date of diagnosis).
References
Agency for Health Care Policy and Research (1992) AHCPR Publication 92-0032. AHCPR.Google Scholar
American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders (4th edn, text revision). APA.Google Scholar
Andrews, E. , Bellard, J. & Walter-Ryan, W. G. (1986) Monosymptomatic hypochondriacal psychosis manifesting as delusions of infestation: case studies of treatment with haloperidol. Journal of Clinical Psychiatry, 47, 188–190.Google ScholarPubMed
Arnold, L. M. (2000) Psychocutaneous disorders. In Kaplan & Sadock's Comprehensive Textbook of Psychiatry (7th edn on CD-ROM) (eds B. I. Sadock & V. A. Sadock), . Lippincott, Williams & Wilkins.Google Scholar
Avnstorp, C. , Hamann, K. & Jepsen, P. W. (1980) Parasitforrykthed behandlet ned pimozid (Orap(R)) [Delusions of parasite infestation treated with pimozide (Orap)]. Ugeskrift for Laeger, 142, 2191–2192.Google Scholar
Aw, D. C. , Thong, J. Y. & Chan, H. L. (2004) Delusional parasitosis: case series of 8 patients and review of the literature. Annals of the Academy of Medicine, Singapore, 33, 89–94.Google ScholarPubMed
Baumer, L. (1951) Die Behandlung des Juckreizes insbesondere beim 'Dermatozoenwahn' mit Elektroschock [The treatment of itching with ECT, specifically in delusional parasitosis]. Hautarzt, 2, 131–132.Google Scholar
Benkert, O. & Hippius, H. (2005) Kompendium der Psychiatrischen Pharmakotherapie (5th edn). Springer.Google Scholar
Bers, N. & Conrad, K. (1954) Die chronische taktile Halluzinose [The chronic tactile hallucinosis]. Fortschritte Neurologie Psychiatrie, 22, 254–270.Google Scholar
Bhatia, M. S. , Jagawat, T. & Choudhary, S. (2000) Delusional parasitosis: a clinical profile. International Journal of Psychiatry in Medicine, 30, 83–91.CrossRefGoogle ScholarPubMed
Bourgeois, M. & Nguyen-Lan, A. (1986) Syndrome d'Ekbom et délires d'infestation cutanée. I. Revue de la littérature [Ekbom's syndrome and delusion of skin infestation. I. Review of the literature]. Annales Medico Psychologiques (Paris), 144, 321–340.Google Scholar
Bourgeois, M. , Rager, P. , Peyre, F. , et al (1986) Fréquence et aspects du syndrome d'Ekbom enquěte auprés des dermatologues francais (A propos de 150 cas) [Prevalence and symptomatology of Ekbom's Syndrome treated by French dermatologists (150 cases)]. Annales Medico Psychologiques (Paris), 144, 659–668.Google Scholar
Charcellay, P. (1843) Annales médico-psychologiques, II, 485 (zitiert nach Griesinger Die Pathologie und Therapie der psychischen Krankheiten [The Pathology and Therapy of Psychological Illnesses]. Krabbe, Stuttgart ().Google Scholar
Chiu, H. F. K. , Ungvari, G. S. & Wong, C. K. (1996) Delusional parasitosis in the elderly: treatment with sulpiride. Clinical Gerontologist, 16, 62–65.Google Scholar
De Leon, O. A. , Furmaga, K. M. , Canterbury, A. L. , et al (1997) Risperidone in the treatment of delusions of infestation. International Journal of Psychiatry in Medicine, 27, 403–409.CrossRefGoogle ScholarPubMed
Driscoll, M. S. , Rothe, M. J. , Grant-Kels, J. M. , et al (1993) Delusional parasitosis: a dermatologic, psychiatric, and pharmacologic approach. Journal of the American Academy of Dermatology, 29, 1023–1033.CrossRefGoogle ScholarPubMed
Ekbom, K. A. (1938) Der praesenile Dermatozoenwahn [Presenile delusional parasitosis]. Acta Psychiatrica Neurologica Scandinavica, 13, 227–259.Google Scholar
Freudenmann, R. W. (2002) Der 'Dermatozoenwahn'. Eine aktuelle Übersicht. [Delusions of parasitosis: an up-to-date review]. Fortschritte der Neurologie Psychiatrie, 70, 531–541.CrossRefGoogle Scholar
Freudenmann, R. W. (2003) Ein Fall von 'Dermatozoenwahn' bei schwerer Herzinsuffizienz. Olanzapin im Rahmen einer multimodalen Therapie. [A case of delusional parasitosis in severe heart failure. Olanzapine within the framework of a multimodal therapy]. Nervenarzt, 74, 591–595.CrossRefGoogle Scholar
Freudenmann, R. W. & Schönfeldt-Lecuona, C. (2005) Delusional parasitosis: treatment with atypical antipsychotics. Annals of the Academy of Medicine Singapore, 34, 141–142.Google ScholarPubMed
Freudenmann, R. W. & Lepping, P. (2006) Dopaminergic antipsychotics in delusional parasitosis (author reply). Progress in Neurology and Psychiatry, 10, 20.Google Scholar
Freudenmann, R. W. , Schönfeldt-Lecuona, C. & Lepping, P. (2007) Primary delusional parasitosis treated with olanzapine. International Psychogeriatrics, .CrossRefGoogle Scholar
Freyne, A. & Wrigley, M. (1994) Delusional infestation in an elderly population. Irish Medical Journal, 87, 86–88.Google Scholar
Freyne, A. , Kenny, E. & Cooney, C. (1999) Delusions of infestation – a case report of response to risperidone. Irish Medical Journal, 92, 435.Google Scholar
Frithz, A. (1979) Delusions of infestation: treatment by depot injections of neuroleptics. Clinical and Experimental Dermatology, 4, 485–488.CrossRefGoogle ScholarPubMed
Gallucci, G. & Beard, G. (1995) Risperidone and the treatment of delusions of parasitosis in an elderly patient. Psychosomatics, 36, 578–580.CrossRefGoogle Scholar
Ganner, H. & Lorenzi, E. (1975) Der Dermatozoenwahn [Delusional parasitosis]. Psychiatrica Clinica, 8, 31–44.Google Scholar
Gould, W. M. & Gragg, T. M. (1976) Delusions of parasitosis. An approach to the problem. Archives of Dermatology, 112, 1745–1748.CrossRefGoogle ScholarPubMed
Hamann, K. & Avnstorp, C. (1982) Delusions of infestation treated by pimozide: a double-blind crossover clinical study. Acta Dermato-Venereologica, 62, 55–58.Google ScholarPubMed
Harbauer, H. (1949) Das Syndrom des 'Dermatozoenwahns' (Ekbom) [The syndrome of delusional parasitosis]. Nervenarzt, 20, 254–258.Google Scholar
Huber, G. (1957) Die coenästhetische Schizophrenie. Fortschritte der Neurologie Psychiatrie, 25, 491–520.Google Scholar
Jördens, J. H. (1801) Entomologie und Helminthologie des menschlichen Körpers oder Beschreibung der Bewohner und Feinde desselben unter den Insekten und Würmern . Grau.CrossRefGoogle Scholar
Kim, C. , Kim, J. , Lee, M. , et al (2003) Delusional parasitosis as 'folie a deux'. Journal of Korean Medical Science, 18, 462–465.CrossRefGoogle ScholarPubMed
Kumbier, E. & Kornhuber, M. (2002) [Delusional ectoparasitic infestation in multiple system atrophy]. Nervenarzt, 73, 378–381.Google Scholar
Le, L. & Gonski, P. N. (2003) Delusional parasitosis mimicking cutaneous infestation in elderly patients. Medical Journal of Australia, 179, 209–210.Google ScholarPubMed
Lepping, P. , Gil-Candon, R. & Freudenmann, R. W. (2005) Delusional parasitosis treated with amisulpride. Progress in Neurology and Psychiatry, 9, 12–16.Google Scholar
Lepping, P. , Gil-Candon, R. & Freudenmann, R. W. (2006) Amisulpride as first line treatment for delusional parasitosis. Progress in Neurology and Psychiatry, 10, 28.Google Scholar
Lindskov, R. & Baadsgaard, O. (1985) Delusions of infestation treated with pimozide: a follow-up study. Acta Dermato-Venereologica, 65, 267–270.Google ScholarPubMed
Lyell, A. (1983) Delusions of parasitosis (The Michelsonecture). British Journal of Dermatology, 108, 485–499.CrossRefGoogle Scholar
Lynch, P. J. (1993) Delusions of parasitosis. Seminars in Dermatology, 12, 39–45.Google ScholarPubMed
Magnan, V. & Saury, M. (1889) Trois cas de cocainisme chronique [Three cases of chronic cocaine abuse]. Comptes rendus des séaances de la Societé de Biologie, 60–63.Google Scholar
Moretti, M. & Varga, G. (2000) Pszichiátriai kórképek börtünetei [Psychosomatic dermatologic symptoms]. Orvari Hetilap, 141, 169–172.Google Scholar
Munro, A. (1978a) Monosymptomatic hypochondriacal psychosis manifesting as delusions of parasitosis. A description of four cases successfuly treated with pimozide. Archives of Dermatology, 114, 940–943.CrossRefGoogle Scholar
Munro, A. (1978b) Two cases of delusions of worm infestation. American Journal of Psychiatry, 135, 234–235.Google Scholar
Munro, A. (1982) Delusional Hypochondriasis. A Description of Monosymptomatic Hypochondriacal Psychosis (MHP). Department of Psychiatry, University of Toronto.Google Scholar
Musalek, M. & Kutzer, E. (1989) Psychiatrische und parasitologische Aspekte des Dermatozoenwahnes [Psychiatric and parasitologic aspects of dermatozoon delusion]. Wiener Klinische Wochenschrift, 101, 153–160.Google Scholar
Musalek, M. , Bach, M. , Gerstberger, K. , et al (1989) Zur Pharmakotherapie des Dermatozoenwahns. Die Bedeutung der Differentialdiagnose für die psychopharmakologische Behandlung von Dermatozoenwahnkraken [Drug therapy of delusional parasitosis. The importance of differential diagnosis for psychopharmacologic treatment of patients with delusional parasitosis]. Wiener Medizinische Wochenschrift, 139, 297–302.Google Scholar
National Institute for Clinical Excellence (2002) Schizophrenia. Core Interventions in the Treatment and Management of Schizophrenia in Primary and Secondary Care. NICE.Google Scholar
Nel, M. , Schoeman, J. P. & Lobetti, R. G. (2001) Delusions of parasitosis in clients presenting pets for veterinary care. Journal of the South African Veterinary Association, 72, 167–169.CrossRefGoogle ScholarPubMed
Pacan, P. , Reich, A. & Szepietowski, J. C. (2004) Erfolgreiche kontrolle von dermatozoenwahn mit risperdal – zwei Fallberichte [Delusional parasitosis successfully controlled with risperidone – two case reports]. Dermatologie und Psychosomatik, 5, 193–195.CrossRefGoogle Scholar
Paholpak, S. (1990) Delusion of parasitosis: a report of ten cases at Srinagarind Hospital. Journal of the Medical Association of Thailand, 73, 111–114.Google Scholar
Perrin, L. (1896) Des névrodermies parasitophobiques [On parasitophobic neurodermal diseases]. Annales de Dermatologie et Syphilographie (Paris), 7, 129–138.Google Scholar
Räsänen, P. , Erkonen, K. , Isaksson, U. , et al (1997) Delusional parasitosis in the elderly: a review and report of six cases from northern Finland. International Psychogeriatrics, 9, 459–464.CrossRefGoogle ScholarPubMed
Reilly, T. & Batchelor, D. (1986) The presentation and treatment of delusional parasitosis: a dermatological perspective. International Clinical Psychopharmacology, 1, 340–353.CrossRefGoogle ScholarPubMed
Riding, J. & Munro, A. (1975) Pimozide in the treatment of monosymptomatic hypochondriacal psychosis. Acta Psychiatrica Scandinavica, 52, 23–30.CrossRefGoogle ScholarPubMed
Safer, D. L. , Wenegrat, B. & Roth, W. T. (1997) Risperidone in the treatment of delusional parasitosis: a case report. Journal of Clinical Psychopharmacology, 17, 131–132.CrossRefGoogle ScholarPubMed
Scheinfeld, N. (2003) Delusions of parasitiosis: a case with a review of its course and treatment. Skinmed, 2, 376–378.CrossRefGoogle ScholarPubMed
Sheppard, N. P. , O'Loughlin, S. & Malone, J. P. (1986) Psychogenic skin disease: a review of 35 cases. British Journal of Psychiatry, 149, 636–643.CrossRefGoogle ScholarPubMed
Slaughter, J. R. , Zanol, K. , Rezvani, H. , et al (1998) Psychogenic parasitosis. A case series and literature review. Psychosomatics, 39, 491–500.CrossRefGoogle ScholarPubMed
Srinivasan, T. N. , Suresh, T. R. , Jayaram, V. , et al (1994) Nature and treatment of delusional parasitosis: a different experience in India. International Journal of Dermatology, 33, 851–855.CrossRefGoogle ScholarPubMed
Takahashi, T. , Ozawa, H. , Inuzuka, S. , et al (2003) Sulpiride for treatment of delusion of parasitosis. Psychiatry and Clinical Neuroscience, 57, 552–553.CrossRefGoogle ScholarPubMed
Thibierge, G. (1894) Les acarophobes [The acarophobics]. Revue Généerale de Clinique et de Théerapeutique, 32, 373–376.Google Scholar
Trabert, W. (1993) Der Dermatozoenwahn. Untersuchungen zur Häufigkeit, Klassifikation und Prognose . Homburg/Saar.Google Scholar
Trabert, W. (1995) 100 years of delusional parasitosis. Meta-analysis of 1, 223 case reports. Psychopathology, 28, 238–246.CrossRefGoogle ScholarPubMed
Trabert, W. (1997) 100 Jahre Dermatozoenwahn [100 years of delusional parasitosis]. Medizinische Welt, 48, 33–37.Google Scholar
Ungvari, G. (1983) Abörférgesség-téboly neuroleptikus kezelése. Ideggy Szemle, 36, 391–397.Google Scholar
Ungvari, G. (1984) Behandlung des Dermatozoenwahns durch Neuroleptika [Treatment of delusional parasitosis with neuroleptics]. Psychiatrische Praxis, 11, 116–119.Google Scholar
Ungvari, G. & Vladar, K. (1984) Pimozid-Therapie des Dermatozoenwahns [Pimozide treatment for delusion of infestation]. Dermatology Monatsschr, 170, 443–447.Google Scholar
Ungvari, G. & Vladar, K. (1986) Pimozide treatment for delusion of infestation. Activitas Nervosa Superior (Praha), 28, 103–107.Google ScholarPubMed
van Vloten, W. A. (2003) Pimozide: use in dermatology. Dermatology Online Journal, 9, 3.Google ScholarPubMed
Wenning, M. T. , Davy, L. E. , Catalano, G. , et al (2003) Atypical antipsychotics and the treatment of delusional parasitosis. Annals of Clinical Psychiatry, 15, 233–239.CrossRefGoogle ScholarPubMed
Willan, R. (1799) Die Hautkrankheiten und ihre Behandlung translated by F. G. Friese Breslau Hirschberg Lissa in Südpreussen: Korn.Google Scholar
Winsten, M. (1997) Delusional parasitosis: a practical guide for the family practitioner in evaluation and treatment strategies. Journal of the American Osteopathic Association, 97, 95–99.CrossRefGoogle ScholarPubMed
World Health Organization (1993) The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. WHO.Google Scholar
Zanol, K. , Slaughter, J. & Hall, R. (1998) An approach to the treatment of psychogenic parasitosis. International Journal of Dermatology, 37, 56–63.CrossRefGoogle ScholarPubMed
Zomer, S. F. , De Wit, R. F. , Van Bronswijk, J. E. , et al (1998) Delusions of parasitosis. A psychiatric disorder to be treated by dermatologists? An analysis of 33 patients. British Journal of Dermatology, 138, 1030–1032.CrossRefGoogle ScholarPubMed
Source: https://www.cambridge.org/core/journals/the-british-journal-of-psychiatry/article/antipsychotic-treatment-of-primary-delusional-parasitosis/0ACC6886165EF9FBEAA851B4467301B4
0 Response to "F22 Delusional Disorder Somatic Type Continuous With Psychosis"
Post a Comment